It has been exhaustively demonstrated that peripheral neural sympathetic activity reflects, and is closely and positively correlated with, central noradrenergic (NA) neural transmission, which is ruled by the locus coeruleus (LC)
During normal basal conditions, adrenal glands supply some 20% to the circulating pool of plasma NA, as expressed by the NA/Ad ratio ³ 5 . Thus, increases or decreases of this NA/Ad ratio parallel enhancement or reduction of the contribution by noradrenergic neural transmission to the global sympathetic activity. According to the above, the neuropharmacological strategy we outlined in order to enhance central NA and peripheral neural sympathetic activities was based on three types of central acting drugs: 1) amino acid precursors of NA (Musgrave, Bachmannet al., 1985; Musso, Brenci et al., 1997); 2) drugs which are able to trigger enhancement of the LC-NA neuronal firing as, for instance, alpha2 antagonists (Rodriguez-Manso, 1999; Verwaerde, Tran et al., 1997; Yan, Jobe et al., 1993), and drugs like buspirone that trigger the firing of NA neurons and, in addition, inhibit serotonergic (5-HT) neurons and disinhibit NA neurons from 5HT-bridling axons (Lechin, van der Dijs et al., 1997b, 1998a; Piercey, Smith et al., 1994); 3) drugs which are able to inhibit the re-uptake of NA by axon terminals and thus prolong the action of NA at the synaptic level (desipramine, protryptiline, nortryptiline, doxepin) (Esler, Wallin et al., 1991; Lechin, van der Dijs et al., 1979b; Sugrue, 1983; Yan, Jobe et al., 1993). Finally, during the last 6 months we frequently prescribed adrafinil or modafinil (alpha-1 agonists) which are very useful substitutes for NA (Anderson and Harvey, 1988; Binder and Powers, 1999; Brustein and Rossignol, 1999; Duteil Rambert et al., 1990; Gerin and Privat, 1998; Jabre and Salzsieder, 1999; Fishman, Feigembaumet al., 1983; Sokoloff, Siegel et al., 1999; Wada, Hasegawa et al., 1997) particularly in some cases where central NA neurons are diminished because of aging. It is very widely known that LC-NA neurons diminish with aging (Arranz, Blennow et al., 1996) and so elder patients may have a limited response capacity to stimulating drugs.
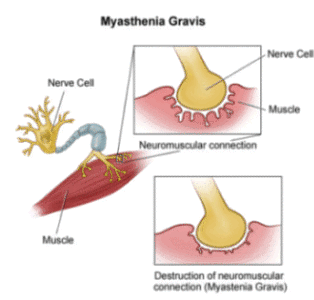
Although other pharmacological manipulations would include drugs that reduce free serotonin in the plasma (f-5HT) like tianeptine, (Lechin, van der Dijs et al., 1998b; 1998c) the results obtained with this tool are not presented in this report. In effect, f-5HT has been shown to block muscular ACh-receptors and so it should play some etiopathogenic role in MG disease, not only by suppressing Th-1 immunity but also by interfering with neuromuscular transmission (Grassi, 1999).
Patients and Methods
Patients
The protocol used in this study was approved by the Ethical Committee of Funda-IME) (Fundación de apoyo al Instituto de Medicina Experimental), Universidad Central de Venezuela, Caracas, Venezuela. All patients signed an informed consent. This was an open study carried out on MG patients recruited from subjects arising from neurology departments in hospitals in Venezuela and other countries. All these patients were referred to our institute because they either did not obtain significant improvement or show progressive impairment. Up to the present, we have enrolled 61 MG patients. However, in this study we included 52 patients only. The remaining nine patients started our therapeutic trial but did not follow it for various reasons: two because of emergency hospitalization early in the trial; five out-of-town patients because of financial difficulties (due to travel expenses despite the fact that our trial, blood tests and drugs were free of charge); and two patients who only attended our institute once. Six patients had received prior treatment in other countries (Columbia Presbyterian Hospital in New York, Jackson Memorial Hospital in Miami, John's Hopkins Hospital in Baltimore, Posbus Hospital in the Republic of South Africa, Mexico City Hospital and the San José Hospital in Costa Rica). Forty-six patients were diagnosed and previously treated in Venezuela: Hospital Universitario in Caracas (24), Hospital Vargas in Caracas (4), Hospital Ruiz y Paez in Ciudad Bolivar (7), Hospital Central in Valencia (4), Hospital Universitario in Maracaibo (2), Hospital Guerra Méndez in Valencia (2), Hospital Militar in Caracas (3).
All patients were diagnosed according to clinical features, electromyography findings and edrophonium testing. Data referring to clinical features are shown in Tables I to V. Methods
Neurochemical Investigation
Plasma neurotransmitters were dosified during basal (supine-resting) condition and after several stress tests: orthostasis, exercise (Lechin, van der Dijs et al. 1995a, 1995b, 1996b, 1996d, 1996e, 1997c), oral glucose (Lechin, van der Dijs et al., 1991, 1992, 1993), and buspirone (Lechin, van der Dijs et al., 1997b, 1998a) according to procedures explained in previously published papers. Blood samples for immunological investigations were processed and transferred to Laboratorios BIOCELL 220, C.A (Caracas, Venezuela) and for some special tests to SPECIALTY LABORATORIES, (Michigan Av., Santa Monica, CA, USA). These routine investigations included: antinuclear antibodies (ANA), cellular immune dysfunction evaluation, CFIDS, AH5O, CH5O, dsDNA autoantibodies, F-actin autoantibodies, humoral immune evaluation, NK cell evaluation, platelet auto-antibodies, Scl-70 autoantibodies, AChR autoantibodies, striational autoantibodies, anti-JO-1, C3/C4, and lymphocyte subpopulations (CD3, CD4, CD5, CD8, CD19, CD2O, CD45RA, CD45RO), immune-globulins (IgA, IgG, IgM, IgE).
Clinical Assessment
We modified the MG scoring system by Osserman and Genking, (1966) by inverting average muscle scoring (AMS) (Mantegazza Antozziet al., 1988; Mendell and Florence, 1990) which provided the numerical score for muscular strength (ocular, facial, abdominal and extremity muscles), and chewing, swallowing, phonation and respiration. Electromyographical parameters plus clinical ratings are expressed together. Scores were verified fortnightly and graded in percentages.
Neurophamacological Therapy
Restoration of central and peripheral NA (alpha-adrenergic) activity:
a) Aminoacid precursors (L-phenylalanine, L-tyrosine), 50 mg before breakfast.
b) Inhibitor of NA re-uptake (desipramine), 25 mg before breakfast.
c) NA-releasing agent + suppressor of serotonergic activity (buspirone) l0-20 mg at 10 am + beta-adrenergic blocking agent (propranolol 10-20 mg).
d) Alpha-1 agonists: adrafinil (150-300 mg) or modafinil (100-200 mg) at 10 a.m.
Go to spinal cord section