Diagnosis Myasthenia
Doctors may suspect MG in anyone with generalized weakness that increases with the use of affected muscles and recovers with rest or in anyone presenting with weakness in the muscles of the eye and face. Since acetylcholine receptors are blocked in MG, drugs that increase the amount of acetylcholine--such as edrophonium--can be used as test drugs, administered intravenously, to see if muscle strength will temporarily improve. Blood testing for antibodies to acetylcholine as well as diagnostic measurement of nerve and muscle function may also be administered. In equivocal cases, electrophysiological studies testing nerve transmission and muscle reaction may be helpful. A computerized axial tomographic (CAT) scan of the chest may reveal an associated thymoma.
SHORT-TERM CONVENTIONAL TREATMENT
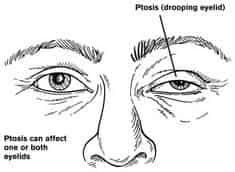
Short-term treatment for MG includes medications to counteract the symptoms of weakness and muscle fatigue.
Anticholinesterases, such as neostigmine and pyridostigmine, which boost the levels of acetylcholine by blocking the enzyme which breaks acetylcholine down, can provide relief for a few hours. Some patients may show no response or even
become weaker while taking the drug. Ephedrine sulfate may be used in conjunction with an anticholinesterase for added strength if patients are not bothered by possible side effects, such as nervousness and insomnia.
Plasmapheresis is an expensive short-term treatment in which several liters of blood are removed from the patient, centrifuged for removal of abnormal antibodies, and returned
intravenously in artificial plasma. This treatment is considered when short-term improvement is crucial for the patient. However, the benefits of the procedure may last only weeks.
High-dose intravenous human immunoglobulin (IVIg) has emerged as a conventional therapy for various neurologic diseases. It may be considered the opposite of plasmapheresis. Rather than expunging the blood of abnormal
antibodies, IVIg floods the body with pooled gamma globulin antibodies from several donors. Although expensive, IVIg has become a first-line or adjunctive therapy in the treatment
of diverse autoimmune diseases, including MG. IVIg therapy has received Food and Drug Administration approval for use as a maintenance treatment of patients with primary humoral (blood-based) immunodeficiencies, and as therapy for acute
or chronic autoimmune thrombocytopenic purpura. In controlled clinical trials, IVIg has been effective in treating chronic inflammatory demyelinating polyneuropathy. IVIg also has produced improvement in some patients with MG, but has had a variable or unsubstantiated benefit in others.
![]()
