IgG
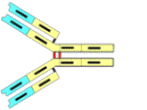
Immunoglobulin G ("IgG") is the main type of the five different types of antibodies found in vertebrates (sharks have only very rudimentary immune factors such as this). "Globulins" are one of five types of protein which are discerned on the basis of their solubility properties.* The antibody molecule is composed of four different polypeptide chains: two larger ones (designated as "H" for heavy) and two smaller chains ("L" for light). Each of these chains (primary structures) are mixes of α-helices and β-pleated sheets, which are the two forms of secondary structure found in the protein world. The four chains are linked together by disulfydryl bonds (cys-cys) as indicated by the dots to make an overall tertiary structure
.
Each H and L chain end in a highly individualized portion (red), which together form pairs that are specific for a type of antigen. Each IgG is thus bivalent and able to attach to two antigenic sites. As each species of the millions of possible types of IgG have different "red" ends, they are called "variable" ("V"). The remainder of the IgG molecules parts (white) are much more uniform from one IgG molecule to another, and are called "constant regions" ("C"). In fact, all the C regions in IgG are identical making IgG what it is: "G." There are two types of C regions in the L chains: κ and λ. Any given molecule of IgG will both L-chains that are identical and thus could be called IgGκ,κ or IgGλ,λ.
Shown here is a slightly more realistic version of how the various sections of the molecule fit together. This schematic was derived from data gleened from X-ray diffraction patterns of crystallized IgG.
It should not be overlooked that the C-regions are highly species specific. In other words, all humans make identical forms, all dogs make another form that differs slightly in primary structure (and the primary structure's impact on the secondary structure). Thus if antibodies from a rabbit are injected into a goat, the goat finds the rabbit antibodies to be foreign and makes antibodies against the rabbit antibodies. While the nomenclature can be confusing, a powerful tool derives from this species specificity and becomes the basis of several analytical assays. For one, ELISA (for Enzyme-Linked ImmunoSorbant Assay) won its developers the Nobel Prize. It is used to detect HIV infection, among many other uses. Now about the nomenclature: The goat's antibodies against zebra antibodies might be called "goat anti-zebra IgG."
IgG is one of five different classes of antibody, each is specified by the type of H-chains it possesses. The five types of H-chain are M, G, A, D, and E. Again, these H-chains are complexed with either κ L-chains or λ L-chains. At first inspection, IgG, IgD and IgE molecules look very much alike, while IgA appears to be a dimer and IgM is a pentamer. "M," by the way, stands for "macroglobulin."
While it is obvious that there are coding sequences in DNA for the constant regions, consideration of the variable regions is far less obvious. It is estimated that the body could possibly make up to 50 million different variable regions, but given that the human genome only has something like 30,000 genes, the question arises: "What codes for the variable regions?" Let your teacher explain this conundrum to you!