Diagnostic value of sural nerve biopsy in chronic inflammatory demyelinating polyneuropathy- Discussion
Four of the six clinical features were more specific than sensitive, as was sural nerve biopsy. Relatively high sensitivity rates were seen in the presence of raised CSF protein concentration and neurophysiological studies consistent with CIDP. A relatively high rate of both sensitivity and specificity was found in neurophysiological studies consistent with CIDP. Our criteria required the demonstration of slowed motor nerve conduction velocities in at least one motor nerve of the arms; if these were present, the values were also abnormal in motor nerves of the legs. The highest positive LRs for CIDP were seen in highly raised CSF protein concentration and neurophysiological studies consistent with CIDP.
Table3 shows the results of two logistic regression models. In the first model all six clinical features were entered. Highly raised CSF protein concentrations, neurophysiological studiesconsistent with CIDP, and, not surprisingly, absence of comorbidity were strong predictors of CIDP. The significant clinical features (p 0.20) as identified from the first logistic model were forced into the second model, as were the conclusions of sural nervebiopsy reports. The same clinical features turned out to be important predictive factors. An independent predictive ability of the sural nerve biopsy could not be shown. In other words, when adjusting for the important clinical features, we could not show that patients with sural nerve biopsy consistent with CIDP were more likely to have CIDP than patients with a negative sural nerve biopsy.
0.20) as identified from the first logistic model were forced into the second model, as were the conclusions of sural nervebiopsy reports. The same clinical features turned out to be important predictive factors. An independent predictive ability of the sural nerve biopsy could not be shown. In other words, when adjusting for the important clinical features, we could not show that patients with sural nerve biopsy consistent with CIDP were more likely to have CIDP than patients with a negative sural nerve biopsy.
DIAGNOSTIC BEHAVIOUR OF THE NEUROLOGIST
The diagnostic performance of the neurologist in terms of within observer reliability based on a subsample of 24 patients was  =0.92 (95% CI 0.85-0.99). The figure shows the results of the neurologists's diagnostic behaviour. The neurologist was ableto discriminate patients with and without CIDP (AUC=0.95; 95%CI 0.90-1.00). His diagnostic performance was not improved anyfurther by offering him additional information about sural nerve biopsy (AUC=0.95; 95% CI 0.90-1.00).
=0.92 (95% CI 0.85-0.99). The figure shows the results of the neurologists's diagnostic behaviour. The neurologist was ableto discriminate patients with and without CIDP (AUC=0.95; 95%CI 0.90-1.00). His diagnostic performance was not improved anyfurther by offering him additional information about sural nerve biopsy (AUC=0.95; 95% CI 0.90-1.00).
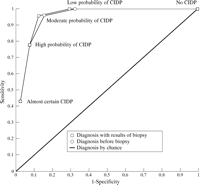
View larger version (19K):
| ROC curves for diagnosis recorded by an experienced neurologist after review of the clinical data before sural nerve biopsy, and diagnosis recorded after review of both clinical data and results of sural nerve biopsy.
|
|
As a consequence of the biopsy reports, the neurologist who reviewed the medical records changed his decision in five patients.The first three patients had CIDP and the last two patients had another diagnosis. Histometry was performed on the biopsies of all three patients with CIDP. Patient I was diagnosed as having almost definite CIDP before biopsy. After biopsy, which showed some abnormalities suggestive of vasculitis, the neurologist changed the diagnosis into moderate probability of CIDP. The evidence for CIDP seemed so strong that the neurologist did not change his diagnosis into no CIDP. Patient II was diagnosed as havinghighly probable CIDP before biopsy. The biopsy, which was alsoanalysed with electron microscopy showed mixed demyelinating and axonal changes and inflammatory infiltrates, which made the neurologist decide to diagnose him as having definite CIDP. Patient III was diagnosed as having CIDP with moderate probability before biopsy and as having CIDP with high probability after biopsy, which had shown mixed demyelinating and axonal changes. Patient IV was diagnosedas having CIDP with moderate probability before biopsy and ashaving CIDP with low probability after biopsy, which had shownaxonal degeneration. Patient V was first diagnosed as having low probability of CIDP. The biopsy, which was also analysed with electron microscopy, showed features of vasculitis, which made the neurologist change the diagnosis into no CIDP.
 | Discussion |
|---|
In this study we investigated the additional diagnostic value of the most invasive diagnostic procedure for the diagnosis of CIDP sural nerve biopsy
sural nerve biopsy when the results of less invasive tests such as medical history, course and distribution of neurological signs and symptoms, and results of blood tests, CSF protein, andneurophysiological studies were already known.
when the results of less invasive tests such as medical history, course and distribution of neurological signs and symptoms, and results of blood tests, CSF protein, andneurophysiological studies were already known.
In the first part of this study, we analysed the objective diagnostic properties of six clinical features and of sural nervebiopsy consistent with CIDP. Neurophysiological studies consistent with demyelination, highly raised CSF protein concentrations and, not surprisingly, absence of comorbidity, were strong predictors for CIDP, whereas this could not be shown for sural nerve biopsy consistent with CIDP.
The objective of the second part of the study was to analyse the diagnostic behaviour of a neurologist experienced in diagnosingperipheral neuropathies. After review of the biopsy reports the neurologist changed his diagnosis in only five of 64 patients. He was able to distinguish between patients with and without CIDP, irrespective of the biopsy data. These results confirm the absence of additional diagnostic value of sural nerve biopsy for diagnosis of CIDP as was statistically shown in the first part of our study.
In all cases the treating neurologist had asked the neuropathologist whether the biopsy was consistent with CIDP. The low diagnostic value of sural nerve biopsy as shown in this study can, therefore, not be explained by the lack of attention in searching for features of inflammatory demyelinating neuropathy. Neither can the results be explained by lack of knowledge of the features consistent with CIDP or the use of inappropriate techniques. All biopsies were investigated after 1989, when the biopsy featuresand techniques to demonstrate inflammatory demyelinating neuropathies were widely known.14
It is difficult to compare the percentage of demyelination found in sural nerve biopsies in our group of patients with CIDPwith that found in other groups of patients with the disease. We relied on the conclusion of the neuropathologist, and foundthat 61% of patients with CIDP had sural nerve biopsies with demyelination. Barohn et al reported predominantly demyelination in 48%, mixed demyelination and axonal changes in 13%, predominantly axonal changes in 21%, and no abnormalities in 18% of sural nerve biopsies of patients with CIDP.17 Krendel et al reported predominantly demyelination in 50% of sural nerve biopsies of 14 patients with CIDP.
Both objective and subjective analyses showed that sural nerve biopsyis a weak diagnostic test. Therefore, sural nerve biopsy is not helpful in confirming the diagnosis of CIDP irrespective of whether there is considerable doubt or almost certainty about the diagnosis of CIDP before biopsy. We conclude that there is no reason to include sural nerve biopsy in research criteria of CIDP, and that sural nerve biopsy has no value in clinical practice to confirmthe diagnosis CIDP before embarking on immunosuppressivetreatment.